Over 100 Women With Unplanned Pregnancy Sue Birth Control Maker Over Error In Packaging
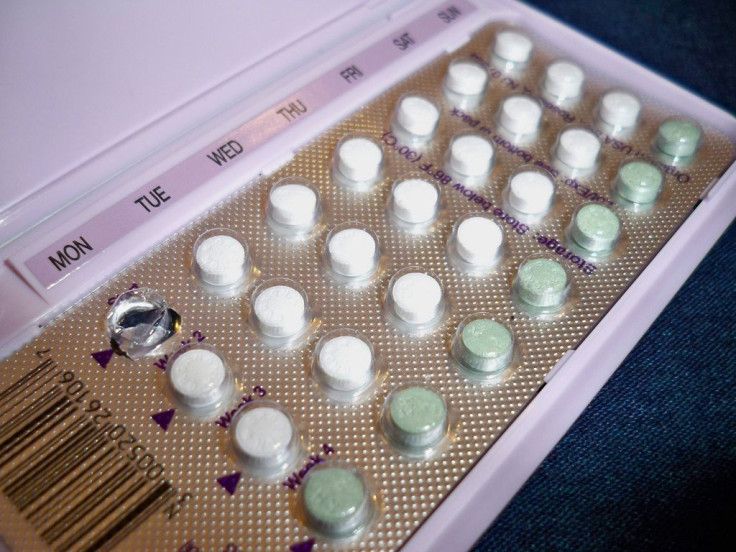
Over 100 women are suing Qualitest Pharmaceuticals, an Alabama-based drug company, for a 2011 error in their birth control's packaging, which they claim resulted in unplanned pregnancies.
Three years ago, Qualitest Pharmaceuticals, a subsidiary of Endo Pharmaceuticals, recalled eight of its birth control pills after discovering the pills’ labeling had been placed upside down. Many women subsequently took the placebo pills when they should have been taking the hormone pills. The lawsuit comprises 94 women who gave birth after taking the defective pills, 17 who didn't carry out their unplanned pregnancies, and two others who did not become pregnant, CNN reported.
There are a number of different types of birth control pills, but they all release one or both of the hormones estrogen and progestin into a woman’s body. These hormones prevent the egg from being released from a woman’s ovary by tricking the body into thinking that it's already pregnant. Some pills also have placebos, or sugar pills, which do not contain any hormones but are meant to be taken at the end of the pill regimen to remind women to take their medication at the same time everyday. A break from the hormones triggers a woman’s monthly cycle.
According to The Planned Parenthood, women are still protected from pregnancy during the days they take the placebo pill as long as they take their medication consistently. This is because the body has already built up an adequate amount of hormones. However, protection against pregnancy is compromised when the pills are not taken consistently, such as when they are taken at different times of the day; when women forget to take them; or, as in this case, when women take the wrong pills.
In addition to incorrectly labeling the pills, the packaging error was also found to hide the lot number and medication’s expiration date, Medical Daily previously reported. Although the Food and Drug Administration announced the defects didn't pose immediate health risks for consumers, it was recommended that consumers begin using a non-hormonal form of birth control immediately, such as a condom, and consult their doctors or pharmacist about the error.
Despite the fact that that company claims to have only been able to confirm one defective birth control packet being sold to a patient, more than 100 women are involved in the class action lawsuit, which was filed last week in a Pennsylvania State Court, CBS reported. The women are seeking millions of dollars in damages to help cover the cost of raising unplanned children into adulthood — which, in 2013, the U.S. Department of Agriculture estimated costs around $245,340.



























