Reducing Chronic Pain: LED Implantable Device Uses Optogenetics To Turn Off Neurons Responsible For Pain
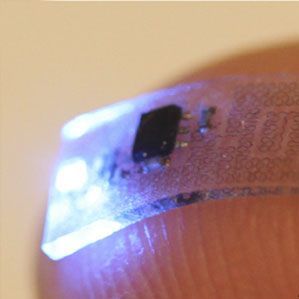
In a world where implants are looking more and more like the way of the future, scientists are still in the beginning stages of creating them. We have already seen paraplegics with electrode implants that help them walk, as well as implants placed on the brains of ALS sufferers, which allows them to control a computer so they can communicate. Now, treating chronic pain may be the next frontier.
In a demonstration published in Nature Biotechnology, researchers displayed a flexible, implantable electronic device that's meant to identify chronic pain and tell users why certain parts of their body are hurting. The end goal of the device is to use an LED to switch chronic pain on and off like you would a lightbulb.
The researchers implanted the device in mice and used it to manipulate neural circuits that are known to be involved in the perception of pain. To do this, the researchers used optogenetics — the manipulation of DNA in neurons that makes them fire or not fire by shining a light on them. Another advantage the researchers displayed was that these devices could be left on for long periods while showing barely any (if any at all) damage to tissue. They also had no effect on motor function.
A further advantage of the implantable device is that it doesn’t need to be stuck to bone to function. The system is made on very soft, thin materials with mechanical functions similar to those found in our body tissue. This is important because it will allow researchers to better understand chronic pain originating from neural activity in both our nervous systems and spinal cords.
Instead of using natural body heat to power the device, it will instead use a tiny, stretchable antenna that takes energy from radio frequency signals. When implanting an earlier device, it was so big that researchers had to put it directly on the mouse’s brain. Now, the smaller device allows for researchers to implant it over the sciatic nerve or in the epidural space — the outermost part of the spinal canal. Using proof-of-concept tests, the researchers were able to display how shining a light on neurons believed to be associated with pain, and modified to be sensitive to light, could make the mice feel pain.
Robert Gereau, a professor of anesthesiology and director of the pain center at Washington University in St. Louis, who started the work on the project, believes this device will allow scientists to better understand how neurons have specific roles when it comes to pain, as well as better understand the type of sensory information that gets processed in the spinal cord. He also believes that optogenetics will help us better understand, treat, and manipulate the way we experience chronic pain.
Source: Gereau, R, et al. Soft, stretchable, fully implantable miniaturized optoelectronic systems for wireless optogenetics. Nature Biotechnology. 2015.



























