Autism Meets Glioblastoma: Novel Strategy For Treating Brain Cancer Discovered In Key Protein
The brains of people with autism may offer scientists a window into the nature of the most common and deadly form of brain cancer, a new study finds. Although applications of the finding are still five to 10 years away, researchers behind the project say the true star of the work may be the unexpected nature of the findings.
“This is a great example of the unexpected good that can come from going wherever the science takes us,” said Dr. Rajini Rao, lead author of the study and professor of physiology at Johns Hopkins University, in a statement.
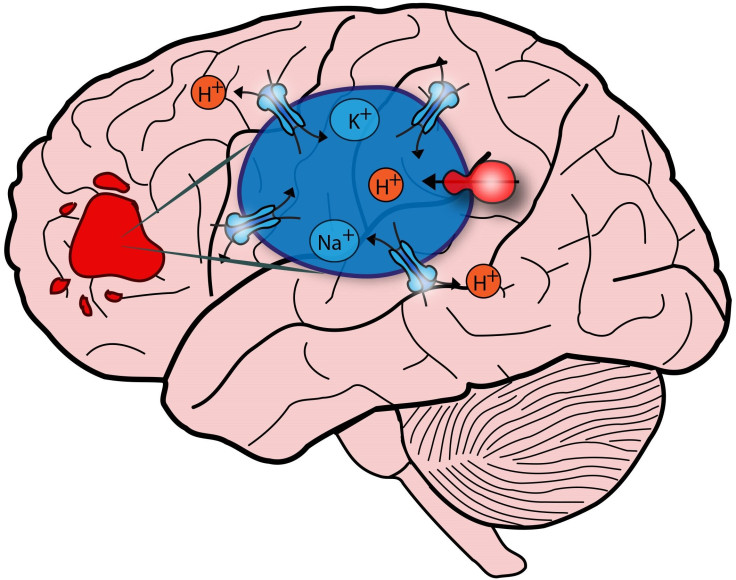
The new findings hinge on a protein called NHE9 and its interaction with the cell’s endosomes. These act as “cargo packages,” ferrying fresh NHE9 in one direction and hauling away old proteins for destruction in the other. Endosomes need to be kept at a certain level of acidity, similar to a leaky bucket that needs to be kept full, Rao says. “Altering either the faucet or the leak rate can dramatically change the water level in the bucket.”
In a 2013 study, she and her colleagues showed autism-associated defects cause NHE9 to destroy proteins too early, as the leaks get “clogged” and the endosomes get too acidic. Now the findings may be applied to a potent form of brain cancer known as glioblastoma, which carries a notoriously low survival rate (10 percent) after five years. The National Cancer Institute estimates roughly 23,000 people suffer from the cancer annually, and around 13,700 of those cases will result in death.
Looking at NHE9 in tumor cells, the research team found greater protein levels were more resistant to radiation treatments and chemotherapy and tended to end in poorer prognoses. Higher levels also predicted faster transport, suggesting quicker metastasis — or cancer growth.
Unlike in autism, however, the team found NHE9 is overrepresented in brain cancer. The cells leak too much and become alkaline. This slows the cancer cells’ transport, causing the harmful proteins to linger on the surface and leave more time to do damage and multiply. In other words, what the team first learned about autism now emerges in the opposite fashion with brain cancer.
One of the primary proteins involved in the cells’ demise is the epidermal growth factor receptor (EGFR). Alkaline endosomes can’t remove EGFR as quickly as healthier endosomes, suggesting the tandem use of drugs that employ NHE- and EGFR-fighting will remove cancer more effectively than ones targeting EGFR alone. Follow-up lab tests confirmed that hypothesis.
“These results are encouraging,” said co-author Dr. Alfredo Quinones-Hinojosa, professor of neurosurgery at Johns Hopkins. “They give us a better idea of what to target so that hopefully we can make this disease less aggressive and less devastating.”
Source: Kondapalli K, Llongueras J, González V, et al. A leak pathway for luminal protons in endosomes drives oncogenic signalling in glioblastoma. Nature Communications. 2015.



























