Biologists Make New Nerve Cells Appear After Brain Injury; Technique Holds Promise As A Neurological Therapy
Researchers at Pennsylvania State University (Penn State) brought us a significant step closer to one day being able to repair the brain. The approach they discovered involves creating neurons where brain injury has occurred by converting another type of cell in the brain into working nerve cells.
"This technology may be developed into a new therapeutic treatment for traumatic brain and spinal cord injuries, stroke, Alzheimer's disease, Parkinson's disease, and other neurological disorders," explained Gong Chen, a professor of biology who headed the study.
The significance of this breakthrough stems from the fact that brain injury and disease usually leads to neurons dying or degenerating. Once damage takes place, the brain has a defense mechanism that kicks in, which involves so-called “reactive glial cells” that protect healthy tissues from succumbing to bacteria and toxins that the injury might introduce. But, oftentimes, this brain response ends up being too much of a good thing by forming scars that prohibit the growth of healthy neurons in the damaged area.
"A brain-injury site is like a car-crash site," Chen explained in a statement. "Reactive glial cells are like police vehicles, ambulances, and fire trucks immediately rushing in to help — but these rescue vehicles can cause problems if too many of them get stuck at the scene.”
Instead of clearing out this traffic jam of glial cells, Chen and colleagues thought it would be wiser to simply convert them to the type of cell that belonged there in the first place: neurons. The idea was straightforward enough but carrying it out was not.
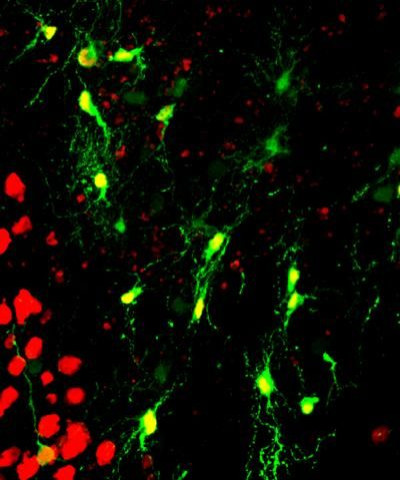
Guided by previous work that managed to reprogram one cell type into another, the team infected the reactive glial cells of healthy mice with a benign virus, which in turn introduced a specific protein gene, NeuroD1. This protein is known to be important in the formation of nerve cells. Within a week of injecting adult mice with this retrovirus, the authors detected two types of glial cells had transformed into neurons. Furthermore, these newly appeared neurons showed promising activity that would allow them to properly integrate into the local brain circuitry.
The researchers also elicited the formation of working neurons in diseased brains of 14-month-old mice, which is the equivalent of a 60-year-old human with Alzheimer’s disease. "Therefore,” Chen observed, “the conversion technology that we have demonstrated in the brains of mice potentially may be used to regenerate functional neurons in people with Alzheimer's disease."
To show this potential, Chen and colleagues tried their technique out on cultured human glial cells. "Within three weeks after expression of the NeuroD1 protein, we saw in the microscope that human glial cells were reinventing themselves; they changed their shape from flat, sheet-like glial cells into normal-looking neurons with axon and dendritic branches," Chen said. His team also found that the new cells exhibited neuron-like behavior by releasing and responding to chemical messengers.
"Our dream is to develop this in vivo conversion method into a useful therapy to treat people suffering from neural injury or neurological disorders," Chen concluded. "Our passionate motivation for this research is the idea that an Alzheimer's patient, who for a long time was not able to remember things, could start to have new memories after regenerating new neurons as a result of our in vivo conversion method and that a stroke victim who could not even move his legs might start to walk again."