Can People Really Die Of Old Age?
The answer is no.
(Alright, thanks for stopping by!)
Just kidding. But really, people don’t die of old age. Though it may seem like a trivial thing to find out, the biology of aging — and the research trailing in its wake — drills down to some pretty profound questions about the nature of existence. But we’re getting ahead of ourselves. First, let’s talk about something more user-friendly, like the inexorable march toward death.
What is aging?
When we talk about aging, we use it in a variety of contexts. The teenager who gets his first zit is hitting puberty — he’s aging physically. The woman who’s forced to install her father in a nursing home — she’s aging mentally. Along the way, all three may also be learning to manage their reactions to these changes, to which we’d say they’re aging emotionally.
Biology would add a fourth kind. As our birthday counters tick upward, our bodies begin to break down. Hangovers don’t fade as quickly as they used to, and suddenly our kneecaps produce a symphony of pops and cracks as we heave ourselves out of bed. These changes punctuate the aging process. We were younger before, and now we’re not — in both attitude and anatomy. We grow into our 80s or 90s, or possibly reach the coveted club of centenarians, and eventually we go. Maybe it’s at the hand of disease. Sometimes we exit quietly. One thing scientists know for sure is that no autopsy report will list “old age” as a cause of death. It just won’t happen. And David Gems has made a living proving why not.
“The idea that people die of pure aging, without pathology, is nuts,” said Gems, the deputy director of the Institute of Healthy Aging and a professor at the University College London. Here Gems uses “pathology” to refer to something that can kill you — some sort of condition, disease, or ailment — not something so boring and normal as having a lot of birthdays. Something else has to be going on.
What isn’t killing us are the processes that define biological aging: telomere shortening, oxidative stress, and glycation. These forces come stock with every human lifespan — congratulations, your body succumbs to entropy — and together they cause old age to be synonymous with old.
As we slog through the fourth dimension, the telomeres that act as protective caps on the end of chromosomes — similar to the aglets on the ends of shoelaces — grow shorter. This leaves the DNA inside exposed to the elements, namely, the other two factors: oxidative stress, which involves the destructive force of oxygen exposure (think nails getting rusty), and glycation, which involves destructive glucose sugar. Both oxidative stress and glycation eat away at the body’s supply of proteins and fats. When those processes pry open the window of our body’s barriers far enough, pathologies are given free rein to slip through, and eventually what results is death.
Being Older (and Braver)
What we non-mutant humans should focus on, today, according to Gems, is that old age as a cause of death is little more than sign of residual confusion, and even self-protection. Aging itself has never contributed to death, but scientists and the people doing science before the profession had a title had no other reasonable explanation for why seemingly healthy people would suddenly drop dead. It wasn’t until later, when high powered scanners and microscopes could see the inner-workings of the brain and other organs, that unknown diseases emerged.
Like the philosophical idea of aging — the notion that everything is hurtling through an unseen dimension, subject to certain physical forces — Gems believes the science of aging bears a similar profundity. In spite of everything, we just need to respect it.
“The problem with aging is that it’s about the most ghastly and tragic aspect of the human condition. Up until recently, there’s been practically nothing one can do about it, so the best thing to do is to lie to oneself about it to make it bearable,” he said, referring to the relative ease of dying from “old age,” rather than crippling pneumonia or the dreaded C-word. “I can’t really blame anyone for that, but as a scientist you have to try to understand things as they really are and hope good things come out of that.”

Can we live forever?
If that conclusion feels anticlimactic, or at best grim, you’re not alone. Scientists and sci-fi buffs alike haven’t been satisfied knowing why we die. The real question, of course, is how we stop dying. If death is just the result of physical breakdown letting in disease, what if the body never breaks down?
Unfortunately, questions like these are hard to answer. Scientists can’t really study other humans to learn how our species ages. We’ve just gotten too good at not dying. Instead, they turn to an organism with a similar biological makeup and one that is perfectly happy living a meaningless, eight-week existence: Caenorhabditis elegans. They’re a species of roundworm. (You might know them better as nematodes.) They’re parasites, and they should be grateful we humans are nice enough to give their lives purpose.
C. elegans are a dream model for a number of reasons. Aside from their short lifespan, their bodies are visually transparent, giving scientists unprecedented access to what’s going on inside. In 2013, for example, Gems and his colleagues at UCL wanted to find out how, exactly, dying happens. Does it hit us like a ton of bricks? Or do we gradually fall out of existence? They discovered the answer fit more with the latter. When the worms died, a fluorescent blue light that the team had injected lit up within the worms’ cells. Over a period of two hours, the blue light intensified. Death propagated through the worms’ bodies and eventually consumed them in a wave of necrosis, or cell death. Even for several minutes after, residual light continued to glow.
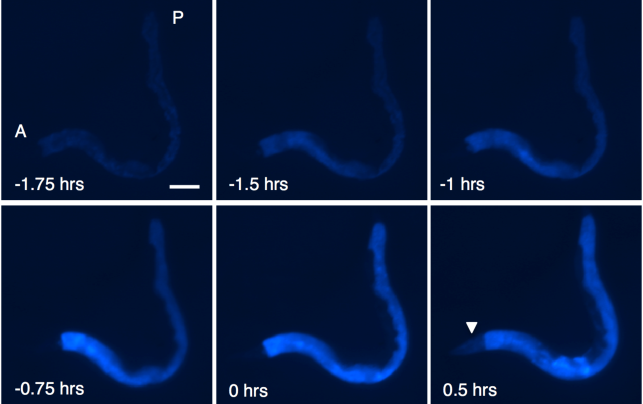
The research is ongoing, if promising. Gems suspects death in humans follows a similar wave-like pattern, though the prospect is at best hypothetical. On the other hand, sometimes hypotheticals are the most fun. Several months after Gems published his study in PLOS Biology, a team of researchers from the Buck Institute on Research and Aging conducted their own study on C. elegans. The goal was to alter the worms’ genes in such a way that the mutation would increase their lifespan by one to two times. What they ended up with was a batch of worms that, in human years, lived well into their 500s.
Lead researcher of the study, Dr. Pankaj Kapahi, said the findings uphold multiple genes as the driving force behind the mutant worms’ impressive feats of aging. We tend to think centenarians — perhaps our own human version of mutants — possess some rare gene that amplifies the healthful effects of diet and exercise. Kapahi’s research says something else. Longevity appeared as the interplay between two genes in particular: one in control of insulin signaling and the other a pathway called Target of Rapamycin, which regulates cell growth, both of which were crammed into a positive feedback loop.
This doesn’t mean altering the same two genes in humans can extend our lives by four centuries. (At least not yet — the science hasn’t been done, so who knows?) Roundworms are faithful models, but they’re still models. To make any sort of credible claim about human longevity, Kapahi would first have to advance the tests to simple mammals, then to mice, then to human cell models, and then if the stars align and ethics panels are feeling sporty that day, maybe, just maybe, he and his team could run tests on humans. That is, if he’s still alive to run them.



























