Family Pleads With Chimerex For Unapproved Drug To Save Boy's Life: How Brincidofovir Could Treat Adenovirus

A 7-year-old boy from Virginia is fighting for his life from his St. Jude’s Children’s Hospital bed in Tennessee, shortly after beating cancer four times since the age of 1. The four-time kidney cancer survivor, Josh Hardy, developed a viral infection — adenovirus — after undergoing a bone marrow transplant in November 2013 for myelodysplastic syndrome, a precancerous bone marrow disorder. After complete heart failure and kidney failure followed the transplant, the need for antiviral medicine became apparent — a life-saving drug a North Carolina pharmaceutical company, Chimerex, is not willing to give.
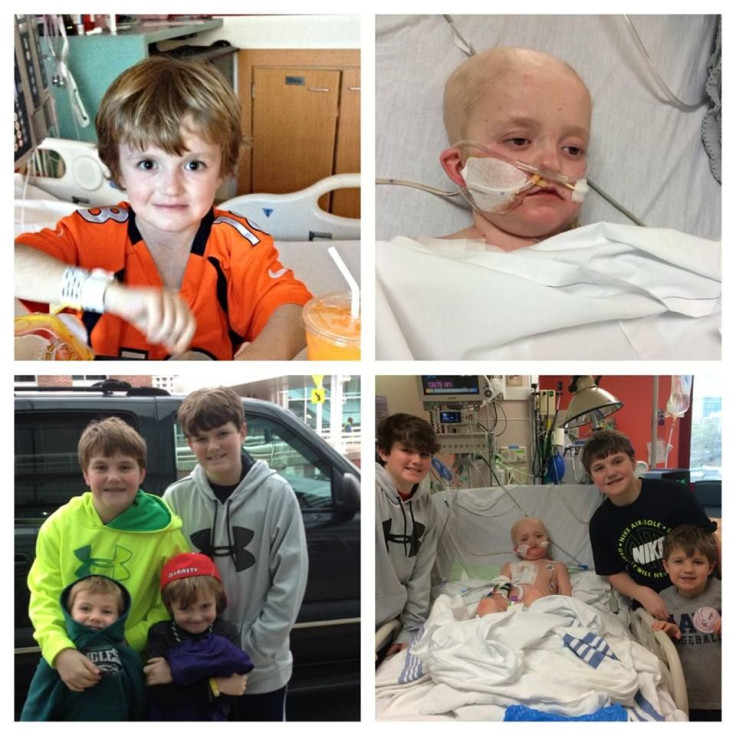
"Having survived four diagnoses of cancer, it would be an absolute travesty for him to meet his demise from a virus," said his mother, Aimee Hardy, to ABC News. "Especially knowing there is a medicine in someone’s hands that can rid of this virus." The drug, brincidofovir, manufactured by Chimerex, has been shown to clear up adenovirus in children in two weeks.
The doctors at St. Jude’s gave Josh antiviral medicine to get rid of the adenovirus he developed after his heart and kidneys began to heal. However, the drug was toxic to the boy’s kidneys, and it wasn’t working. As an alternative, the team of doctors suggested brincidofovir, because they tried it in children in a previous study. "Our doctor said 'This drug will cure and save your son,'" said Josh’s father, Todd Hardy.
Chimerex President and CEO Kenneth Moch, aware that Josh’s life is in his hands, simply said they “can’t afford” to take that risk. The drug company has previously given patients emergency access to medication, but they stopped doing so since it was costly, despite receiving $72 million in federal funding to develop brincidofovir. Moch believes saying yes to Josh would mean saying yes to more patients, and therefore, drowning the company’s resources and delaying FDA approval that would help a greater number of patients. "We all have great compassion for this child," Moch said to ABC News. "We spent our lives trying to develop new medications for patients just like Josh. ... We need to make sure to get this drug available as soon as possible to as many people as possible."
The company participates in compassionate use — the use of medications that have not been approved by the Food and Drug Administration but are in the earlier stages of development. The FDA only allows companies to provide drugs to some patients under certain circumstances. Currently, Chimerex can only give drug approval to patients who have been on the drug in a previous trial and newborns with herpes simplex virus. Josh does not meet either of the criteria.

“It’s horrible for us as parents to see, because he’s a vibrant, strong little boy, and even though he is frail, he has a very strong will about him. But things just keep stacking against him, and we just want to do everything we can to give him the opportunity to make a full recovery,” Aimee told Fox News. Brincidofovir has shown broad-spectrum antiviral activity against all five families of viruses that affect humans, including the adenovirus, and has no evidence of kidney or bone marrow toxicity, according to Chimerex’s website. The adenovirus, although similar to the common cold, can lead to mild and serious complications, such as damage in the eyes, GI tract, bladder, and most notably, a lung problem like acute respiratory distress syndrome, which could be fatal.

Despite Chimerex’s “no,” the Hardys, just like their son, will fight this through and not take “no” for an answer. The family has started to use social media to ask supporters to call (919) 806-1074, email compassionateuserequest@chimerix.com, or tweet Chimerex @chimerex using the hashtag #savejosh. They can even track Josh’s progress via the Save Josh Facebook page. To make a donation to Josh, supporters can also visit his CaringBridge page and have the opportunity to see photos of Josh, and sign the guestbook. To sign the petition for the release of brincidofovir for compassionate use to treat the 7-year-old, click here.



























