FDA-Approved At-Home Stool Screening Test Could Prevent 60% Of Colorectal Cancer Deaths
The U.S. Food & Drug Administration (FDA) today announced the approval of a DNA screening test for colorectal cancer — the first at-home test that can detect red blood cells and DNA mutations in stool to help catch abnormal growths.
Cancer screening is a good way to catch it early, and can significantly reduce one’s risk of developing the disease. The Centers for Disease Control and Prevention (CDC) states that if everyone over the age of 50 had regular screening tests, up to 60 percent of colorectal cancer deaths could be avoided. This is why the new at-home, easy-to-use screening test will be a positive breakthrough.
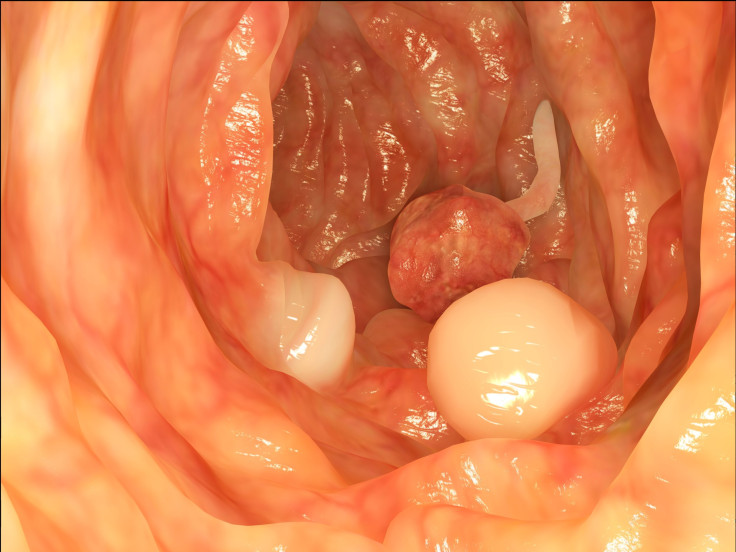
Colorectal cancer is the third most common type of cancer that affects both men and women, and it mostly strikes people over the age of 50. It can occur in the colon (large intestine), as well as the rectum. Typically, colorectal cancer begins as a polyp — which is a flat tissue growth on the walls of the large intestine or rectum. Cologuard, the name of the screening test, works by detecting hemoglobin and certain mutations linked to colorectal cancer as stool moves through the intestines.
In a clinical trial that reviewed 10,023 people, the FDA found that Cologuard was quite accurate — it was able to detect over 92 percent of colorectal cancers and 42 percent of advanced adenomas, or tumors, among the participants. However, people are still advised to use traditional methods of colorectal cancer screening, such as sigmoidoscopy, colonoscopy, or fecal occult blood testing.
“This approval offers patients and physicians another option to screen for colorectal cancer,” Alberto Gutierrez, director of the Office of In Vitro Diagnostics and Radiological Health at the FDA Center for Devices and Radiological Health, said in a press release. “Fecal blood testing is a well-established screening tool and the clinical data showed that the test detected more cancers than a commonly used fecal occult test.”
According to the press release, the Centers for Medicare & Medicaid Services (CMS) also approved the screening test, and CMS will cover Cologuard once every three years for Medicare beneficiaries who meet the following criteria:
- age 50 to 85 years,
- asymptomatic (no signs or symptoms of colorectal disease including but not limited to lower gastrointestinal pain, blood in stool, positive guaiac fecal occult blood test or fecal immunochemical test), and
- average risk of developing colorectal cancer (no personal history of adenomatous polyps, of colorectal cancer, or inflammatory bowel disease, including Crohn’s Disease and ulcerative colitis; no family history of colorectal cancers or an adenomatous polyp, familial adenomatous polyposis, or hereditary nonpolyposis colorectal cancer).



























