FDA Approves Breathe Technologies' Lighter Sleep Apnea CPAP Device [VIDEO]
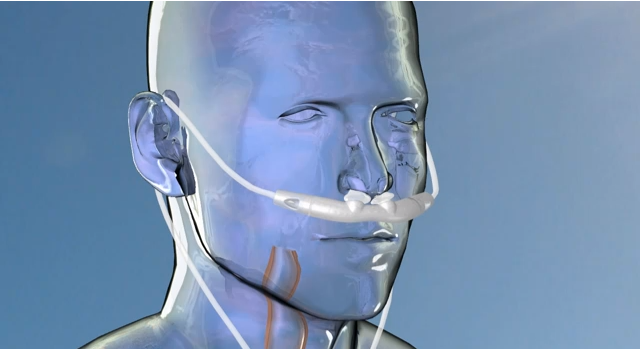
Good news for those who suffer from obstructive sleep apnea, a condition that prevents people from taking regular breaths while sleeping and reduces oxygenation in the body. The U.S. Food and Drug Administration has approved Breathe Technologies' Sleep System for marketing. One in every 15 Americans is affected by sleep apnea and up to 24 percent of middle aged men and 9 percent of women are undiagnosed and untreated.
The Sleep System (Continuous Positive Airway Pressur or CPAP) is meant to treat patients with obstructive sleep apnea, in which interruptions in breathing during sleep are caused by blocking of the airways. The new device is smaller than previous systems, offers a new humidification system, and operates with a user-friendly, touch-screen interface. The device works by synchronizing itself to the patient's breathing patterns while sleeping, pressurizing oxygen into the nose with each inhale.
The smallness and portability of the new device is considered a breakthrough, as previous models are so cumbersome that many patients would rather go without the potentially life-saving device.
"Reaching this milestone ahead of schedule is a testament to the expertise of our world-class team," said Larry Mastrovich, CEO of Breathe Technologies. "Our approach to respiratory care is centered on improving patients' quality of life. Based on extensive research with patients and clinicians, we have designed a clinically effective system with unique features."
Symptoms of obstructive sleep apnea include daytime sleepiness and fatigue caused by disturbed sleep patterns during the night. One study even linked sleep apnea to an increase in cancer risk because the lack of oxygen causes blood vessel growth, which can help tumors to grow. Obstructive sleep apnea is also implicated in a 4.8 fold increase in cancer mortality. Sleep apnea is also associated with other diseases such as cardiovascular disease, stroke, high blood pressure, arrhythmias, diabetes, and sleep-deprived driving accidents. A major risk factor of obstructive sleep apnea is obesity, because physical changes with weight gain make the airway more susceptible to occlusion.
Current treatments for sleep apnea include surgery to correct physical issues that prevent airway opening during sleep, or devices that keep the airway open such as special mouthpieces which keep the mouth open and jaw slightly forward. Pharmacological treatment is available through acetazolamide (Diamox), which lowers blood pH to force the body to breathe more often.



























