FDA Approves Procysbi To Treat Rare Cellular Disease
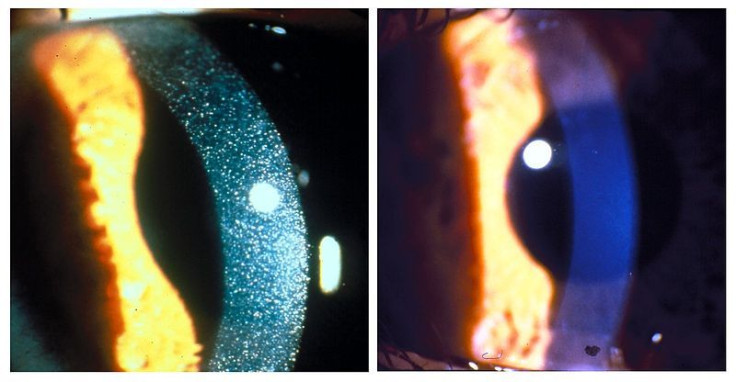
A rare genetic disease that depletes nutrient levels in children may have finally met its match with the new drug Procysbi, approved by The Food And Drug Administration (FDA) to treat the uncommon disorder called nephropathic cystinosis.
The National Institutes of Health (NIH) has been encouraging researchers and companies to explore "orphan diseases," syndromes that affect very few people but have no current treatment options. Large pharmaceutical and biotechnology companies are also motivated by the fact that if treatments are found, insurance companies will pay large amounts of money to treat these rare cases.
Only 500 people in the United States and 3,000 people around the world are affected by cystinosis. The illness, resulting from an amino acid called cystine (a cousin to the sleep-inducing, turkey-derived tryptophan), is fatal if there is no intervention during childhood. Excess cystine can result in issues with kidney function and allow too much salt, sugar, and nutrients to exit the body through urine, robbing the individual of valuable nourishment.
Children who have the disease grow slowly and have brittle bones due to the lack of nutrition, even if they can eat a balanced diet.
A drug called cystagon was approved in 1994 and also treated cystinosis. The old drug required patients to take a dose every six hours, while the new medication can be consumed every 12 hours.
"Procysbi is the only delayed-release product approved by FDA to treat nephropathic cystinosis, offering patients with this rare disease an important new treatment option," said Andrew E. Mulberg, deputy director of the FDA's Division of Gastroenterology and Inborn Errors Products.
The kicker is that the new drug costs $250,000, according to a Raptor Pharmaceutical press release. This is compared to $8,000 for the current treatment.
NIH established the Office of Rare Diseases Research in 1993 with the goal of identifying, stimulating, coordinating, and supporting research and responding to the needs of patients who have any one of the 7,000 rare diseases known today.
In 2011, NIH invested more than $3.5 billion dollars toward 9,400 rare disease research projects. Of that amount, nearly $750 million went toward more than 1,600 orphan drug research projects.
Published by Medicaldaily.com



























