First Do-It-Yourself HIV Test Moves Closer to FDA Approval
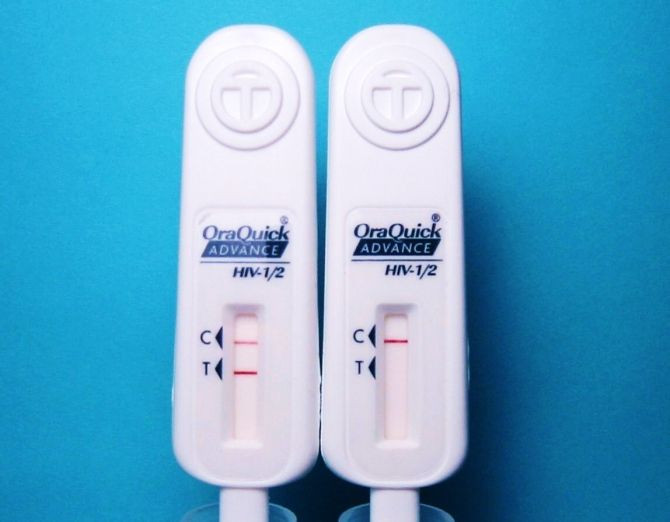
Members of a federal advisory panel Tuesday unanimously recommended that the U.S. Food and Drug Administration approve the first over-the-counter HIV test for at-home use, in a 17-0 vote.
The panel concluded that OraSure Technologies Inc.'s mouth-swab test, which is currently sold commercially to health professionals to be used at facilities, is safe and effective for determining whether a person has the AIDS virus.
Members of the panel said that the test, which can detect the presence of antibodies to the virus in less than 20 minutes, has the ability to prevent new HIV infections and provide HIV-positive people with access to medical care and social services, outweighs the risks of false results.
The panel’s recommendations will now be considered by agency regulators as they determine whether the OraQuick In-Home HIV Test, should be approved as first over-the-counter in-home HIV test.
While the FDA is not required to follow the panel’s recommendations, it usually does.
If federal regulators approve, it would be sold in pharmacies like tests for pregnancy and blood sugar.
One of the members of the advisory panel, Dr. Steven Pipe of the University of Michigan said that the new test will reduce the number of people who never get tested for HIV, especially those who are at risk for the infection.
"I can't get past the quarter of a million people in the U.S. who have HIV and are not tested," Pipe said at the meeting, according to WebMD. "If we make any dent in that, the answer is yes, we realize the [OraQuick At-Home] benefit outweighs its risks."
The price of the at-home test have yet to be determined, and experts predict that over-the-counter tests will cost more than the $20 professional version, partly because it will come with a instruction booklet and because OraSure will establish a call center with trained counselors that are available 24 hours a day, seven days a week.
"The price will be substantially lower than $60," OraSure executive vice president and chief science officer Stephen Lee, told the committee.
However, in clinical trials researchers found that the at-home test failed to detect 7 percent of participants with HIV infection, with a professional test that happened only 2 percent of the time.
Researchers are still not sure whether the negative results were because of the home test or if it was because people read the test incorrectly.
Nonetheless, the FDA predicts that an extra 45,000 people will correctly learn they have the virus in the first year after OraQuick is approved for home use.
There are about 50,000 new HIV infections every year, according the U.S. Centers for Disease Control and Prevention.
The CDC says that about 1.2 million Americans have HIV, and nearly 20 percent of those who are HIV-infected don't know they carry the virus.



























