The IUD Makes A Contraceptive Comeback: Is An Intrauterine Device Right For You?
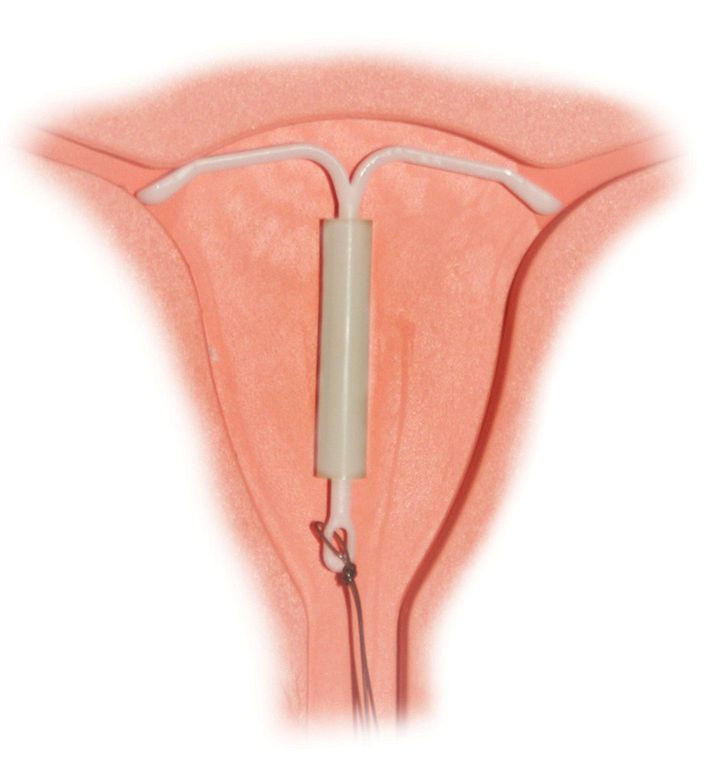
Despite being one of the most effective forms of birth control, intrauterine devices, or IUDs for short, are not widely used in the United States. But public and women’s health experts say it’s time for the form of contraception to make a comeback.
More than 99 percent effective, IUDs are small T-shaped devices inserted by a practitioner into the uterus. The devices prevent pregnancy by keeping eggs from implanting in the walls of the uterus. The two most popular brands in the U.S. are the ParaGard, which is made out of copper and lasts for up to 10 years, and the Mirena, which is plastic, lasts for up to five years, and also releases progestin, one of the hormones present in certain contraceptive pills.
The advantages of IUDs over other methods are many. IUD users need not worry about remembering a daily pill or using proper technique. Unlike barrier methods such as condoms and diaphragms, IUDs are discrete and do not interfere with the spontaneity of sex.
Pelvic Inflammatory Disease And The Dalkon Shield
But the barriers to widespread adoption of IUDs are plentiful too. Most notably is the negative legacy left behind by the poorly designed Dalkon Shield, an earlier version of an IUD released in 1971. The Dalkon Shield introduced bacteria into the uterus, leading to many cases of pelvic inflammatory disease. Pelvic inflammatory disease (PID) is an infection of the uterus, fallopian tubes, or other reproductive organs, and serious cases could result in infertility, ectopic pregnancies, or chronic pelvic pain.
After reports of infections, some leading to septic abortions, became widespread, the Food and Drug Administration advised women to have the Dalkon Shield removed in 1983. Ensuing lawsuits forced the A. H. Robins, the device’s manufacturer, to declare bankruptcy in 1985.
But with a new generation of young women exploring contraceptive options, the Dalkon Shield is a figment of a past never experienced. Newer IUDs are designed to limit the risk of PID, which is small but still exists. Bayer Healthcare Pharmaceuticals, manufacturer of the Mirena IUD, says PID develops in less than 1 percent of users.
Costs Of IUDs And Risk Of Uterine Perforation
Another barrier to the adoption is provider training. Physicians and nurse practitioners must be taught how to properly insert the device into the uterus, an uncomfortable procedure which carries the risk of perforating the uterus. Perforation occurs in about 1-2 times in every 1,000 insertions, with risk being higher among inexperienced practitioners.
Other downsides to IUDs include side effects, including irregular bleeding and painful cramping. Also, the initial cost of the device and insertion could be several hundred dollars upfront, although over the long term, this cost could be less compared to other forms of contraception. IUDs, along with other forms of contraception, should be covered by insurance plans, thanks to a provision in the Affordable Care Act.
While the IUD is completely reversible and women can get pregnant soon after, users need to make an appointment to take it out.
Women's Health Advocates And Manufacturers Push For More IUD Use
Global health workers with NGOs such as Jhpiego and Saving Lives at Birth have been pushing for greater IUD use abroad, where spacing out pregnancies is a health priority. IUDs are especially useful for mothers who recently gave birth, as some do not give off hormones that could interfere with breastfeeding.
Healthcare providers are now pushing for greater IUD use in domestic markets too. Last year the American College of Obstetricians and Gynecologists recommended that IUDs "should be offered as first-line contraceptive options for sexually active adolescents.” Previously, IUDs were marketed mainly at first-time mothers. This might be explained by providers' tendency to recommend them to women in monogamous relationships, since IUDs alone do not protect against sexually transmitted diseases. However, IUDs could be used together with condoms that do offer protection.
Market forces and new products also are bringing more public attention to IUDs. Skyla, an IUD similar to the Mirena with a smaller dose of hormones, was released earlier this year. Industry insiders predict more products in the future.
"I think we're also going to see in the future other alternative devices," said Dr. Jeffrey Peipert, a professor of obstetrics and gynecology at Washington University, in an interview with NPR. "I think we're going to see this market expand."
Published by Medicaldaily.com



























