New Sickle Cell Disease Treatment Eliminates Need Of Immunosuppressants After Stem Cell Transplant
A new stem cell transplant that reverses sickle cell disease (SCD) is being touted as a major breakthrough in treatment options, as it enables patients to stop the lifelong use of anti-rejection drugs after a transplant. The study conducted at the National Institutes of Health's Clinical Center in Bethesda, Md., by researchers from NIH's National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) and the National Heart, Lung, and Blood Institute was published Tuesday in the Journal of the American Medical Association.
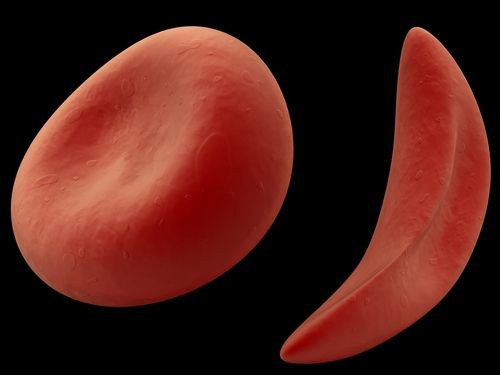
SCD is a debilitating condition that occurs due to a mutation in the red blood cells that causes them to take a crescent or sickle shape. This disrupts the red blood cells' ability to effectively carry oxygen in the body. Due to less oxygen, the patient experiences several complications like pain, anemia, or even organ failure.
Blood and marrow stem cell transplants have seen considerable success in reversing SCD. But introducing the marrow of a donor results in rejection of the graft by the patient’s immune system or graft-versus-host disease. To overcome these side-effects, patients are given immunosuppressants, which may have to be taken lifelong.
But in this new trial, the patients, most of them with severe SCD, were given a modified blood stem cell transplants. The transplant not only reversed the sickle cell, but also eliminated the need to use immunosuppressants. This was achieved by only partially replacing the bone marrow as most adults with sickle cell could not handle the chemotherapy regimen needed to completely destroy their own bone marrow before replacing it with a donor’s.
They were then given low doses of radiation and immunosuppressants. This allowed the donor stem cells to move in and produce healthy blood cells. The donor stem cells were taken from healthy siblings. It effectively reversed SCD in 26 of 30 patients and allowed them to achieve stable mixed donor chimerism, a condition in which a person has two genetically distinct cell types in the blood. Some of these patients had participated in a 2009 study by NIH, where partial stem cell transplants had reversed SCD in nine out of 10 patients.
Fifteen of 30 adults then stopped taking the medication under careful supervision one year after transplant and still had not experienced rejection or graft-versus-host disease at a median follow up of 3.4 years. "Side effects caused by immunosuppressants can endanger patients already weakened by years of organ damage from sickle cell disease," said Dr. John F. Tisdale, the paper's senior author and a senior investigator at NIH, in a press release. "Not having to permanently rely on this medication, along with use of the relatively less-toxic partial stem-cell transplant, means that even older patients and those with severe sickle cell disease may be able to reverse their condition."
"Typically, stem-cell recipients must take immunosuppressants all their lives," said Dr. Matthew Hsieh, lead author on the paper and staff clinician at NIH. "That the patients who discontinued this medication were able to do so safely points to the stability of the partial transplant regimen."
Sickle cell patients often experience "relentless pain," Hsieh added. "Following the transplant, we saw a significant decrease in hospitalizations and narcotics to control that pain."
This new approach gives hope to the 90,000 or so people estimated to have SCD. It is an inherited, lifelong disease found more commonly in people of African ancestry.
"The devastating complications associated with sickle cell disease can deeply affect quality of life, ability to work and long-term well-being," said NIDDK Director Dr. Griffin P. Rodgers, a co-author on the paper. "This study represents an important advance in our efforts to make a potentially transformative treatment available to a wider range of people, especially those who could not tolerate a standard stem-cell transplant or long-term use of immunosuppressants."
Source: Tisdale J, Hsieh M, Rodgers G. Journal of the American Medical Association. 2014.



























