Scientists Isolate Human Egg-Producing Stem Cells, Aging Women To Make More Eggs?
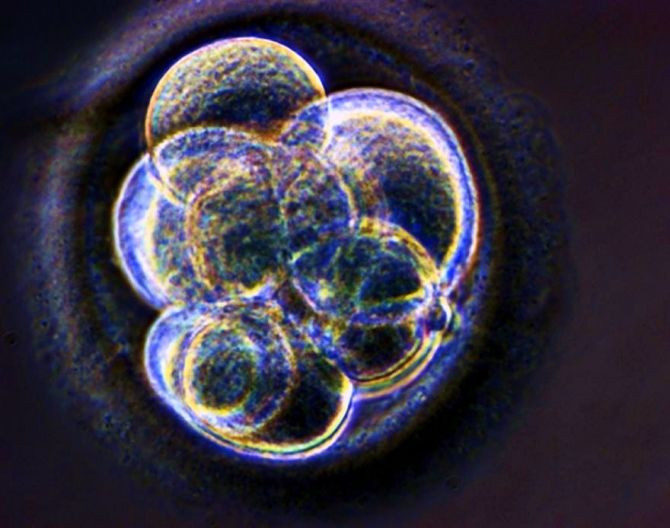
Scientists have discovered egg-producing stem cells in the ovaries of young women that can be transformed into immature eggs, according to a new study that was published on Sunday in the journal Nature Medicine.
Doctors have long believed that women were born with a number of eggs that will only deplete as they age, but Harvard researchers said that the discovery could offer potential new developments for fertility treatments for women who are infertile because of disease or age.
"Our current views of ovarian aging are incomplete. There's much more to the story than simply the trickling away of a fixed pool of eggs," said lead researcher Jonathan Tilly of Harvard's Massachusetts General Hospital, in a statement released on Sunday by Massachusetts General Hospital.
Tilly and his team of researchers reported that they’ve identified and isolated the stem cells that are able to produce eggs in the ovaries of women of reproductive age, by looking for a unique protein located on the surface of stem cells called the DDX4 protein, which allowed them to filter out the right cells.
The latest experiment is a follow up to Tilly’s 2004 paper which was also published in Nature, which had initially drew fierce skepticism, but was later supported by other studies that demonstrated that female mice that underwent oocyte-destroying chemotherapy were able to have successful pregnancies after receiving bone marrow or blood cell transplants.
Researchers from another study published in 2009 in Nature Cell Biology, isolated and cultured egg-producing stem cells from adult mice and transplanted the immature stem cells into chemotherapy-treated infertile female mice, which then became mature enough to be ovulated, fertilized and developed into healthy offspring.
"That study singlehandedly deflated many of the arguments from critics of our earlier Nature paper by showing that oocyte-producing stem cells exist in mice and could develop into fully functional eggs," said Tilly.
In an experiment on living human ovarian tissue that had been grafted inside mice, Tilly said that the cells had "spontaneously generated" immature eggs - or oocytes that was identical to oocytes in the body.
"The primary objective of the current study was to prove that oocyte-producing stem cells do in fact exist in the ovaries of women during reproductive life, which we feel this study demonstrates very clearly,” Tilly said in a statement.
"The discovery of oocyte precursor cells in adult human ovaries, coupled with the fact that these cells share the same characteristic features of their mouse counterparts that produce fully functional eggs, opens the door for development of unprecedented technologies to overcome infertility in women and perhaps even delay the timing of ovarian failure," Tilly added.
Many fertility experts said that Tilly’s study was exciting because it had showed quite convincingly there were stem cells in the ovaries of women that make eggs.
Tilly and his team did not try to make the eggs in his latest experiment fully functional because it is illegal in the U.S. to experimentally fertilize human eggs
"In this paper we provide the three key pieces of evidence requested by those who have been skeptical of our previous work," Tilly said.
"We developed and extensively validated a cell-sorting protocol to reliably purify OSCs from adult mammalian ovaries, proving once again that these very special cells exist. We tested the function of mouse oocytes produced by these OSCs and showed that they can be fertilized to produce healthy embryos. And we identified and characterized an equivalent population of oocyte-producing stem cells isolated from adult human ovaries," he concluded.
“If Dr. Tilly can reverse the biologic clock or halt it, and start making eggs from stem cells, it’s fantastic,” says Avner Hershlag, MD, chief of the Center for Human Reproduction at North Shore University Hospital in Manhasset, N.Y.
“This is the true reproductive emancipation of women,” he told WebMD. “You will be free to a) not compromise on who you share your life and share your kids with, and b) like any man, you will have the freedom to develop a full professional life and not have to stop everything because you are having children.”
However, Hershlag believes that this sort of fertility advancement for women might still be years away.
“Her best bet is actually, right now, to freeze her eggs if she wants to delay reproduction,” he said about women looking to buy more time to have a baby.
Investigators are currently exploring whether oocyte stem cells can also be frozen and then retrieved when a woman wants to have a baby. The researchers said that while human eggs are extremely delicate and could be spoiled when frozen and thawed, the same risk would not apply to the egg-producing cells.



























