Stem Cell Therapy Proves Effective As Treatment For Lung Disease In Preterm Infants
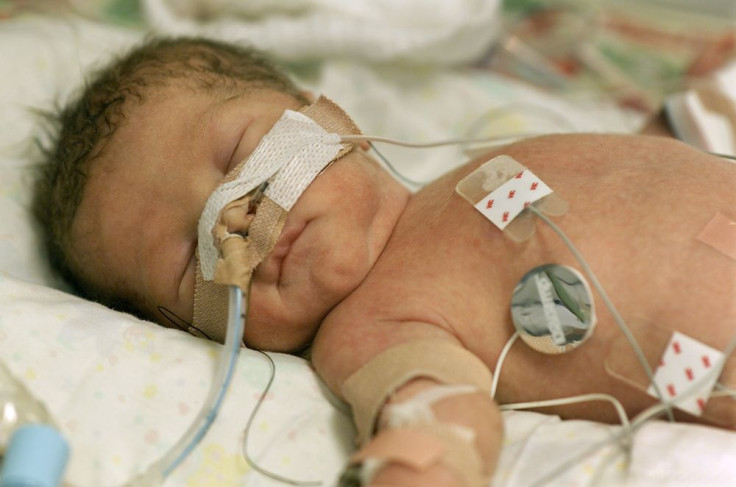
Babies who are born very preterm — at less than 32 weeks of pregnancy — run an increased risk of developing a chronic lung disease known as bronchopulmonary dysplasia (BPD). Looking to treat the fragile infants who develop this condition, researchers have found that stem cell therapy could be effective.
Advances in medical science have led to a rise in preterm births, as the ability to keep these infants — sometimes born at less than 25 weeks — alive has improved. Almost 500,000, or one in eight, babies is born premature every year, according to the Centers for Disease Control and Prevention (CDC). Each one of them is at risk for health problems, with respiratory and breathing conditions among the most common because their lungs haven’t fully developed.
Preterm infants who are born more than 10 weeks before their due dates, weigh less than 2 lbs., and have breathing problems, are most at risk of developing BPD, although they aren’t immediately diagnosed with it, according to the National Institutes of Health. First, they must be diagnosed with respiratory distress syndrome, a condition caused by their lung’s inability to produce enough surfactant, a substance that coats the membranes and allows the lungs to expand and contract. The deficiency forces the baby to breathe through a ventilator while undergoing oxygen and surfactant therapy. If they reach their due date and still need oxygen therapy, they’re diagnosed with BPD.
Researchers from the Samsung Medical Center and Biomedical Research Institute in Seoul, South Korea, found that umbilical cord blood-derived mesenchymal stem cells could prevent this diagnosis in infants born with a high risk of BPD. These cells, which haven’t developed into specialized cell types yet, could be used to repair damaged or underdeveloped organs, like these infants’ lungs. The researchers conducted a Phase I trial looking at how these cells affected the development of BPD in nine infants born between 24 and 26 weeks.
Every one of the infants responded well to the procedure without any serious side effects. Only 33 percent — three of them — developed moderate BPD and none developed severe BPD. By comparison, 72 percent of a matched control group developed moderate to severe BPD. The treatment also seemed to reduce the risk of another problem that arises from preterm birth, retinopathy of prematurity, which occurs when blood vessels in the eye develop abnormally.
With success in these trials, the study’s lead author, Dr. Won Soon Park, said in a statement that “these findings strongly suggest that phase II clinical trials are warranted to test the efficacy of mesenchymal stem cell transplantation, which could lead to new therapies to prevent or cure BPD.” In the meantime, the researchers are conducting a long-term follow-up study on the nine infants, testing for safety.
Source: Park W, Chang Y, Ahn Y, et al. Mesenchymal Stem Cells for Bronchopulmonary Dysplasia: Phase 1 Dose-Escalation Clinical Trial. The Journal Of Pediatrics. 2014.



























